Range of usage
This method is suitable for a mass content of aluminum between 0.1-5.0% in natural iron ores, iron ore concentrates and agglomerates, including sinter products.
Introduction
Iron ore samples are dissolved in hydrochloric acid and nitric acid. The solution is then evaporated to dehydrate silica, followed by dilution and filtration. Afterwards, the residue is ignited and the silica in the sample is removed by evaporation with hydrofluoric and sulfuric acids. Then, the residue is fused with sodium carbonate and the cooled melt is dissolved in the filtrate. Then, the solution obtained is aspired into the flame of atomic absorption spectrophotometer, using nitrous oxide-acetylene flame atomization. The content of aluminum is measured at 396.2nm wavelength, using Al hollow cathode lamp and calculated in reference to the Al standard solution.
Equipment and Reagent
Equipment and apparatus:
1. PERSEE atomic absorption spectrophotometer A3F, equipped with nitrous oxide/acetylene gas supply & air/acetylene gas supply. PERSEE aluminum hollow cathode lamps.
2. Muffle furnace, durable of maintaining a temperature of 1100C
3. Platinum crucible, applicable with the muffle furnace
Reagents (analytical grade):
1. Sodium carbonate (Na2 CO3), anhydrous
2. Hydrochloric acid, ρ 1. 19g/ml;
3. Nitric acid, ρ 1.4g/ml;
4. Hydrochloric acid (1+9) ρ 1.19 g/ml solution: diluted 1+9;
5. Hydrofluoric acid, ρ 1. 13g/ml, 40% mass fraction, or ρ 1. 185g/ml, 48% mass fraction
6. Sulfuric acid, ρ 1.84g/ml, diluted 1+1;
7. High purity iron, purity (mass) >99.9%, with a aluminum content of <0.002%;
8. High purity aluminum, purity (mass) >99.9%
9. Background solution:
a) Dissolve 10g of high purity iron in 50 ml of hydrochloric acid and oxidize by adding nitric acid drop by drop (as less as possible);
b) Evaporate until a syrup-like sticky solution is obtained.
c) Add 20 ml of hydrochloric acid and dilute to
200 ml with water.
d) Dissolve 17 g of sodium carbonate in water and add it to the iron solution in last step.
e) Transfer the solution to a 1000 ml volumetric flask and dilute to volume with water.
Aluminum standard solution:
1. Aluminum standard stock solution (Al content 500μg/ml):
Dissolve 0.5000 g of high purity aluminum in 25 ml of hydrochloric acid. Let cool and transfer the solution to a 1000 ml volumetric flask. Add water to mark and mix solution well.
2. Aluminum calibration solutions (of aluminum content of 0; 5.00; 12.5; 25.0; 50.0; 100; 125 μ g/ml):
a) Transfer 2.0; 5.0; 10.0; 20.0; 40.0 and 50.0 ml portions of [Aluminum standard stock solution (Al content 500μg/ml)] into 200 ml volumetric flasks.
b) Dilute each flask with water to about 100 ml.
c) Add 6 ml of hydrochloric acid and 60 ml of background solution to each flask.
d) Prepare a blank aluminum calibration solution by transferring 60 ml of background solution to a 200 ml flask and add 6 ml of hydrochloric acid.
e) Dilute all solutions to 200 ml with water and mix.
Sample treatment:
1. Dried test sample preparation:
a) For eligible sample material, use sample of particle size less than 100 μm, which has been prepared in accordance with ISO 3082.
b) Take a representative portion of sample from the sample material as test sample.
c) Dry the test sample at 105 ±2 C.
2. Decomposition of the test portion
a) Take several increments: weigh approx. 1g of dried test sample.
b) Transfer the test portion to a 250 ml beaker. Moisturize it with a few ml of water. Add 25 ml of hydrochloric acid, cover with a
watch-glass and heat gently. Increase the heat and digest just below boiling for a few minutes.
c) Add 2 ml of nitric acid and digest for a several minutes.
d) Remove the watch-glass and keep the solution boiling for 30min (105- 110C), evaporating most ofthe solution.
e) Add 5 ml of hydrochloric acid to dissolve the residue. Put the watch-glass back on the beaker and keep warm for several minutes.
f) Add 50 ml of water and heat solution to boiling, wash the watch-glass and walls of the beaker.
g) Then, filter the solution through a medium-texture paper into a 250 ml beaker.Carefully remove all adhering particles with a rubber-tipped rod or moistened filter paper.
h) Wash three times with hydrochloric acid, then wash with hot water until the filter paper is free of yellow color.
i) Transfer the paper and residue to platinum crucible. Evaporate the filtrate to about 100 ml and retain it.
3. Treatment of the residue
a) Ignite the paper and residue in the platinum crucible at 500 to 800 C.
b) Cool, moisten with a few drops of water. Add 3 to 4 drops of sulfuric acid and 10 ml of hydrofluoric acid.
c) Evaporate slowly to expel silica.
d) Remove excess sulfuric acid by heating it up and keeping it at about 700 C (acid will evaporate in white smoke).
e) Add 1.0 g of sodium carbonate to the residue, covering the crucible and fuse at 1100 C for 15 min over a burner or in a muffle until a clear melt is obtained.
4. Preparation of the test solution
a) Dissolve the cooled melt residue in the retained filtrate, then remove and wash in the crucible and cover.
Note: if the solution is cloudy at this stage, indicating the presence of hydrolyzed titanium. It should be filtered prior to next step.
b) Transfer the solution to a 200 ml flask, dilute with water and mix. Inject this solution directly to the atomic absorption spectrophotometer as testing solution.
Note: if the mass fraction of aluminum in the original sample is greater than 2.5%, then, transfer a 40 ml aliquot to a 200 ml volumetric flask, add 50 ml of background solution and 4 ml of hydrochloric acid. Dilute to volume with water and mix. Inject this solution instead as testing solution.
Instrumental preparation:
1. Apply Al hollow cathode lamp to the A3F atomic absorption spectrophotometer. Preheat for 30min.
2. Adjust lamp energy to 100%
3. Set wavelength to 396.2 nm, search peak to make sure that the lamp and instrument is in normal functioning.
4. Ignite with air-acetylene for 10 min. Then, switch to nitrous oxide-acetylene.
5. Inject the aluminum standard solution of the highest aluminum content. Adjust gas flow and burner head height to obtain the maximum absorbance.
6. Then, inject water and select [auto zero].
Equipment Condition
Instrument Working Parameters:
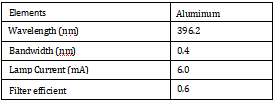
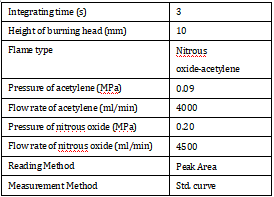
Table 1. Instrument Working Parameters.
Experiment Procedure
Standard Curve of aluminum and running
Run [standard aluminum solution of aluminum content of 0; 5.00; 12.5; 25.0; 50.0; 100; 125μg/ml] and then make a standard curve using the results.
Run samples prepared above and calculate the results using AAWIN software.
Calculation:
The mass fraction of aluminum,wAl , expressed as a percentage, is calculated to four decimal places using
Formula below:
![]()
where:
ρAl is the mass concentration, in micrograms per ml, of aluminum in the final test solution; m is the mass, in grams, of sample contained in 200 ml of the final test solution