Introduction
With the change of geography and geology, as well as the factors of pollution, the quality of water has changed greatly. Therefore, whether it is consumer water or industrial water, it is extremely important to understand the metal content of water.
Although there are many kinds of technologies to determine minerals in water. The simplest, cheapest and fastest technology is atomic absorption spectrometry. Therefore, although ICP-OES and ICP-MS are increasingly popular, AAS is still widely used.
This experiment focuses on the determination of seven non-toxic elements in drinking water by Persee A3flame atomic absorption spectrometer. Although other low content elements can also be determined by flame atomic absorption spectrometer, the most common method is using graphite furnace atomic absorption spectrometer, ICP-OES or ICP-MS.
Experimental
Sample and Standard Preparation
Water samples are taken from urban and local wells. Mineral water is purchased from local grocery stores. In addition, there are water samples certified to meet drinking water standards. (trace metals in drinking water – high purity, Charleston, South Carolina, USA).
The sample was prepared by adding 1% nitric acid (V / V) and 0.1% lanthanum chloride as releasing agent for the determination of calcium and magnesium, and as ionization inhibitor for the determination of sodium and potassium.
Under the conditions of Table 1 and Table 2, the samples were analyzed by A3 flame atomic absorption spectrometer. Due to the high content of minerals in water samples, the combustion head was rotated 30 ° to suppress the signal intensity, so as to meet the needs of mineral analysis.
In addition, the determination of potassium and sodium using emission mode, A3 can automatically optimize the experimental conditions, broaden the range of analysis, so even if the content is high can also be determined. This mode can reduce the dilution ratio of the sample in the determination of potassium and sodium.
The sample is introduced into the system by self absorption through the standard high sensitivity atomizer. In the determination of copper, iron and zinc, the nebulizer does not use a spacer (which provides maximum sensitivity). A spacer was used for the determination of sodium, potassium, magnesium and calcium.
Instrumentation
Table 1. A3 determination conditions of all elements
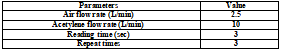
Table 2. A3 analysis conditions for each element
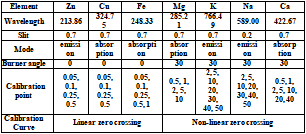
Results
The correlation coefficient of all calibration curves is 0.999 or better. The accuracy of the calibration curve was evaluated by an independent calibration verification (ICV) solution diluted 100 times to fall within the calibration curve. Table 3 shows the measurement results of ICV and the accuracy of calibration curve.
Table 3. ICV Test result.
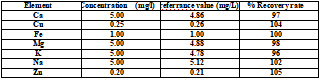
In order to verify the reliability of the method, the reference sample was determined at first, and the determination results are shown in Table 4. The recovery rate fluctuates within 10% of the standard value, which fully demonstrates the accuracy of the method.
Table 4 . Reference sample test result (mg/L)
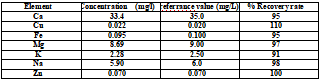
Table 5 . Sample test result (mg/L)
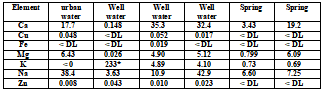
Sample needs to be diluted 10 times.
Through the established method, several drinking water samples in each area were determined. City water and well water are collected directly from taps, and mineral water is poured directly from bottled water purchased. The results are shown in Table 5.
Copper and zinc are detected in four samples taken from the tap, which may be leached from copper pipes, fittings and solder.
Further investigation shows that the resident has installed a water softener with K as counterion to remove high content of calcium and magnesium in well water.
As expected, copper and zinc were not detected in the spring, only minerals were detected. The change of mineral concentration shows the different geological characteristics of the water source.
Finally, the blank solution was determined ten times, and the detection limits of copper, iron and zinc were obtained by calculating the standard deviation of three times. (i.e. 1% HNO3) results are shown in Table 6. Due to the high content of mineral elements, the detection limits of these elements (such as potassium, calcium, sodium and magnesium) were not determined. In addition, the content of these elements is usually high, so the detection limit is meaningless for them.
Table 6. Detection limit
![]()
Conclusion
This work shows that A3 has the ability to successfully determine mineral elements in drinking water samples, including urban water, well water and spring water. Trace elements and mineral elements can be determined by rotating the combustion head and adopting the emission mode of the instrument. The Syngistix touch software on the instrument can operate the A3atomic absorption spectrometer completely through the touch screen interface.
Of course, you can also run AAWin software by connecting to a computer. This flexibility makes A3 the best choice for drinking water analysis.