แมงกานีส (Mn) เป็นองค์ประกอบธรรมชาติที่พบในแม่น้ํา ทะเลสาบ และน้ําพื้นผิวอื่น ๆ มันช่วยในปริมาณเล็ก ๆ แต่เป็นอันตรายเมื่อมีมากเกินไป โรงงานที่ใช้น้ำพื้นผิวสําหรับการทำงานหรือการปล่อยของเสียต้องตรวจสอบระดับแมงกานีสอย่างใกล้ชิด นี่ช่วยให้พวกเขาอยู่ภายในกฎสิ่งแวดล้อม และช่วยให้การดำเนินงานของพวกเขาดําเนินการอย่างราบรื่น สเปคโตรโฟโตเมตรีการดูดซึมอะตอม (AAS) เป็นเครื่องมือที่แม่นยำมากสําหรับการค้นหาแมงกานีส จุดแม้กระทั่งปริมาณเล็กน้อย ๆ สุดลงไปยังระดับส่วน
ความสำคัญของการติดตามแมงกานีสในน้ำอุตสาหกรรม
การติดตามปริมาณแมงกานีส เป็นสิ่งสำคัญมากสําหรับการปฏิบัติตามกฎ การปกป้องธรรมชาติ และรักษาการทำงานโรงงานให้มีประสิทธิภาพ
ผลกระทบต่อสุขภาพและสิ่งแวดล้อมของแมงกานีสเกิน
แมงกานีสเกินไปในน้ำดื่มหรือโรงงานอาจทําให้เกิดปัญหา ในคน โดยเฉพาะอย่างยิ่งเด็ก ระดับแมงกานีสสูง อาจทำร้ายการทำงานของสมอง ในธรรมชาติ แมงกาเนียมมากเกินไป อาจเป็นอันตรายต่อปลาและสิ่งมีชีวิตในน้ำอื่น ๆ ที่ทำให้ความสมดุลของระบบนิเวศเสียหาย
มาตรฐานกฎหมายสําหรับระดับแมงกานีสในน้ำ
องค์กรสุขภาพโลกกำหนดจำกัด 0.1 มก. ลิตร ⁻ ¹ (1.8 μM) สำหรับแมงกานีสในน้ำดื่ม สําหรับเสียจากโรงงาน จำกัดมักจะอยู่ตั้งแต่ 0.05 ถึง 0.3 มก. ลิตร ⁻ ¹ ขึ้นอยู่กับกฎหมายท้องถิ่น โรงงานจึงต้องการเครื่องมือที่ดีมาก เพื่อวัดแมงกานีสอย่างแม่นยำ
ความท้าทายในอุตสาหกรรมในการจัดการมลพิษแมงกานีส
โรงงานที่ใช้น้ำพื้นผิวมักจะเผชิญกับปัญหา เพราะระดับแมงกานีสเปลี่ยนแปลง การเปลี่ยนแปลงเหล่านี้มาจากฤดูกาล ฝนน้ำไหล หรือขยะจากแหล่งขึ้น หากโรงงานไม่มีระบบที่ดีในการตรวจสอบและบำบัดน้ำ การเปลี่ยนแปลงเหล่านี้อาจทำลายเครื่องจักร คุณภาพผลิตภัณฑ์ต่ำกว่า หรือให้เกิดการละเมิดกฎ
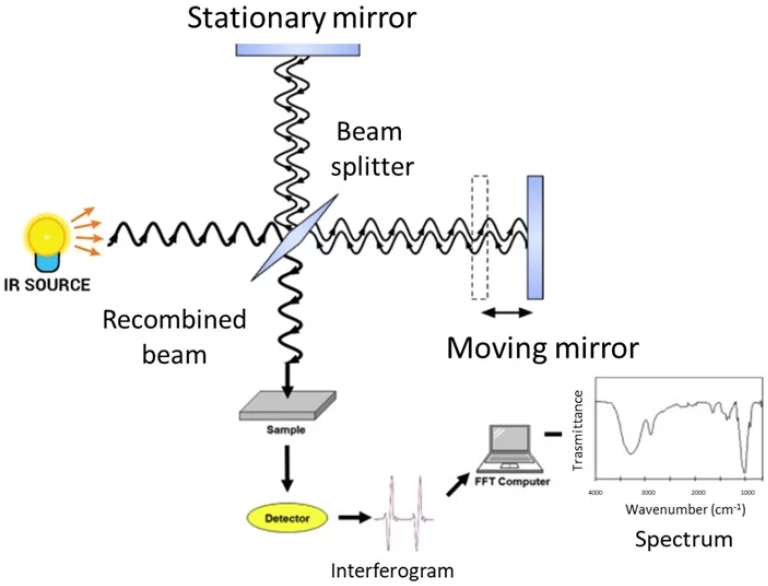
หลักการการดูดซึมอะตอม Spectrophotometry (AAS)
AAS เป็นวิธีที่มีความไวสูงสุด ในการวัดโลหะขนาดเล็ก ๆ เช่นแมงกานีส ในผสมที่ซับซ้อน เช่นน้ำพื้นผิว
วิธีการวัด AAS ติดตามโลหะในตัวอย่างน้ำ
สำรวจภูมิศาสตร์สหรัฐอเมริกาได้กําหนดขั้นตอนที่ชัดเจนสําหรับการทดสอบน้ํา ตามที่แสดงในกระดาษ 1549-C (1966) และกระดาษ 1540-G (1966) ขั้นตอนเหล่านี้จะทำให้แน่ใจว่าการตรวจจับโลหะน่าเชื่อถือ AAS ทํางานโดยการเปลี่ยนตัวอย่างน้ำเป็นอนุภาคเล็ก ๆ จากนั้นมันจะวัดแสงเท่าไหร่ที่อนุภาคเหล่านี้ดูดซึมในความยาวคลื่นบางอย่าง นี่เป็นไปตามกฎ Beer-Lambert ที่ปริมาณแสงที่ดูดซึมแสดงให้เห็นว่ามีโลหะมากแค่ไหน
ส่วนประกอบสำคัญของ Spectrophotometer การดูดซึมอะตอม
การรู้ว่าแต่ละส่วนทํางานอย่างไรช่วยให้แน่ใจว่าการวัดถูกต้อง
แหล่งแสงและ Monochromator
โคมไฟแคโทดกลวงที่อุ่นเป็นเวลา 30 นาที ส่งแสงที่ 275.6 นาโนเมตร (2756 Å) สําหรับแมงกานีส โมโนโครเมเตอร์เลือกแสงนี้ ทําให้องค์ประกอบอื่น ๆ ไม่ทําให้ผลลัพธ์ถูกเสียหาย
Atomizer และระบบการแนะนำตัวอย่าง
ตัวอย่างน้ำจะเข้าสู่เตาไฟหรือเตากราไฟต์ เพื่อแยกมันเป็นชิ้นเล็ก ๆ โดยการเปลี่ยนผสมของน้ำมันเชื้อเพลิงและอากาศในเครื่องเผาไหม้น้ำมันเชื้อเพลิง พนักงานสามารถสร้างไฟได้ที่อากาศหนักหรือเชื้อเพลิงหนักขึ้นอยู่กับสิ่งที่ต้องการ
เครื่องตรวจจับและโปรเซสเซอร์สัญญาณ
เครื่องตรวจจับสัญญาณแสง สัญญาณนี้ถูกเพิ่มขึ้น และส่งไปยังระบบ ที่แสดงหมายเลขบนหน้าจอ ตัวเลขนั้นเท่ากับปริมาณโลหะในตัวอย่าง
วิธีการตรวจจับแมงกานีสโดยใช้ AAS
เพื่อให้ได้ผลลัพธ์ที่ดีเมื่อทดสอบแมงกานีสด้วย AAS พนักงานต้องปฏิบัติตามขั้นตอนอย่างระมัดระวังจากการเก็บตัวอย่างจนถึ
เทคนิคเก็บตัวอย่างและการเก็บรักษา
ตัวอย่างน้ำต้องเก็บไว้ในภาชนะที่สะอาดซึ่งจะไม่รั่วไหลโลหะ มันถูกกรองผ่านเมมเบรน 0.45 ไมโครม เพื่อหลีกเลี่ยงการอุดตันเครื่อง atomizer หลังจากเก็บรวบรวมคนงานเพิ่มกรดไนตริก (HNO) ₃) เพื่อให้ตัวอย่างมั่นคง
การเตรียมมาตรฐานการปรับเทียบสําหรับการวิเคราะห์ Mn
มาตรฐานสําหรับการทดสอบแมงกานีสทำดังนี้:
- โซลูชั่นมาตรฐาน Iความร้อน 0.5 กรัม MnSO ₄·H₂ O ที่ 120 ° C เป็นเวลาหนึ่งชั่วโมง ผสมในน้ำบริสุทธิ์ 100 มิลลิตรกับ H 1 มิลลิตร ₂SO₄ และฟอร์มาลิน 5 มิลลิตร จากนั้นเพิ่มน้ำเพิ่มเติมเพื่อให้ 100 มิลลิตร (1.00 มิลลิตร = 0.105 มก. แมงกานีส)
- โซลูชั่นมาตรฐาน II: ใช้โซลูชั่นมาตรฐาน I 10 มิลลิตร และเพิ่มน้ำเพื่อให้ 100 มิลลิตร (1.00 มิลลิตร = 0.0105 มก. แมงกานีส)
- มาตรฐานการทำงาน: ทำชุดของสารละลายจาก 0.00 ถึง 1.0 มก. ลิตร ⁻ ¹ โดยการเพิ่มน้ำใน Standard Solution II เหล่านี้ใช้ในการวาดกราฟเพื่อเปรียบเทียบกับตัวอย่างที่ไม่รู้จัก
เงื่อนไขการทำงานสำหรับการตรวจจับ Mn ที่แม่นยำด้วย AAS
หลายสิ่งที่ต้องตั้งค่าเพื่อให้ได้ผลลัพธ์ที่ดี:
การเลือกความยาวคลื่นสำหรับการตรวจจับแมงกานีส
แสง 275.6 นาโนเมตร (2756 Å) ถูกเลือกสําหรับแมงกานีส มันชัดเจนซุปเปอร์และหลีกเลี่ยงการผสมผสานกับองค์ประกอบเช่นเหล็กหรือแมกนีเซียม
การใช้เทคนิค Flame vs. เตากราไฟต์ AAS
Flame AAS ทำงานได้ดีสำหรับระดับแมงกานีสสูงกว่า 1 มก. ลิตร ⁻¹. สำหรับระดับต่ำกว่า 1 มก. ลิตร ⁻ ¹ เตากราไฟต์ AAS (GFAAS) ดีกว่า GFAAS สามารถจุดเพียง 0.01 มก. ลิตร ⁻ ¹ ด้วยการขยายขนาด 10x ทําให้มันแม่นยํามาก
ปัจจัยที่ส่งผลต่อความแม่นยำของการวัด Mn ด้วย AAS
แม้ว่าจะมีการตั้งค่าที่ดีที่สุด บางสิ่งที่อาจทำให้การวัดผิดพลาด
การแทรกแซงแบบแมทริกซ์และกลยุทธ์การจ่ายตัวอย่าง
แมกนีเซียมไม่ทําให้เกิดปัญหาเมื่อองค์ประกอบอื่น ๆ เช่นโซเดียมอยู่ในตัวอย่าง แต่ถ้ามีของแข็งมากเกินไป หรือบิตที่ละลาย มันอาจทำให้ผลไม่ชัดเจน การเพิ่มน้ำเพื่อบางตัวอย่างหรือใช้วิธีพิเศษที่เรียกว่าการเพิ่มมาตรฐานสามารถช่วยได้ การเพิ่มมาตรฐานเป็นเรื่องดีเมื่อของแข็งในน้ำทำให้ยากที่จะตรงกับมาตรฐานน้ำบริสุทธิ์
การปรับเทียบเครื่องมือและการควบคุมคุณภาพ
ตัวอย่างทุกกลุ่มต้องการการทดสอบว่าง การทดสอบซ้ำ ตัวอย่างที่มีจุด และการตรวจสอบด้วยมาตรฐานที่รู้จัก ขั้นตอนเหล่านี้ทําให้ผลลัพธ์น่าเชื่อถือ เนื่องจากการได้ผลเดียวกันทุกครั้งเป็นเรื่องยาก คนงานจึงสร้างกราฟใหม่สําหรับตัวอย่างแต่ละกลุ่ม
การบำรุงรักษาประจําเพื่อให้มั่นใจว่าเครื่องมือมีเสถียรภาพ
การรักษาเครื่องในรูปร่างที่ดีเป็นกุญแจ คนงานควรทำความสะอาดเผา แลกเปลี่ยนโคมไฟเก่า ตรวจสอบการไหลของก๊าซ และรีเซ็ตเครื่องบ่อยครั้ง ขั้นตอนเหล่านี้ทําให้ผลลัพธ์มั่นคง
เทคนิคในการกำจัดแมงกานีสจากน้ำพื้นผิว
การหาแมงกานีสไม่เพียงพอ โรงงานต้องเอามันออกก่อนที่จะปล่อยหรือใช้น้ำใหม่
การใช้โพแทสเซียม Permanganate หรือคลอรีนเป็นออกซิเดนต์
โพแทสเซียม permanganate (KMnO) ₄) เป็นสารเคมีที่พบมากที่สุดสำหรับการกำจัดแมงกานีส คนงานเพิ่มมันระหว่างขั้นตอนการบำบัดอากาศและการกรอง นี่ทำให้จับแมงกานีสง่ายขึ้น
สื่อกรองเหมาะสำหรับการกำจัด Mn
ทรายละเอียด มีขนาดระหว่าง 0.55 และ 0.75 มม. ทํางานได้ดีเมื่อต้องถอดแมงกานีสเท่านั้น ทรายนี้จับแมงกานีส โดยไม่ติดอุดตันเกินไป
วิธีการแลกเปลี่ยนไอออนและการกรองเมมเบรน
วิธีการที่แฟนซี่ เช่น การแลกเปลี่ยนไอออน และการกรองเมมเบรน สามารถเลือกแมงกานีส แต่มักจะต้องมีขั้นตอนเพิ่มเติมเพื่อเตรียมน้ำ ขึ้นอยู่กับสภาพเริ่มต้นของมัน
การรวม AAS ในกระบวนการทำงานบำบัดน้ำอุตสาหกรรม
การใช้ AAS ในงานโรงงานประจำวันช่วยให้มีการเลือกที่ฉลาดในโรงบำบัดน้ำ
การติดตามในเวลาจริงในโรงงานบำบัดโดยใช้ AAS
AAS ปกติต้องใช้เวลาในการเตรียมตัวอย่างดังนั้นจึงไม่ได้ทันที แต่เครื่องใหม่สามารถใช้ตัวอย่างได้ไม่หยุด นี่ให้ข้อมูลอัปเดทอย่างรวดเร็วเกี่ยวกับระดับแมงกานีสระหว่างการรักษา ช่วยให้คนงานทํางานอย่างรวดเร็ว
การบันทึกข้อมูลและการวิเคราะห์แนวโน้มสําหรับการปรับปรุงกระบวนการ
เครื่อง AAS ใหม่ทํางานกับโปรแกรมคอมพิวเตอร์ เพื่อบันทึกผลลัพธ์ คนงานสามารถดูผลเหล่านี้ได้ในเวลาที่ผ่านมา โดยการจุดรูปแบบพวกเขาสามารถปรับสารเคมีเช่น KMnO ได้ ₄ หรือคลอรีน สิ่งนี้ช่วยให้ระดับแมงกานีสอยู่ภายในขอบเขตที่ปลอดภัย และทําให้การทํางานมีประสิทธิภาพมากขึ้น
PERSEE: ผู้ผลิตเครื่องมือวิเคราะห์ที่เชื่อถือได้
บริษัท Beijing Purkinje General Instrument Co., Ltd.เริ่มต้นในปี 1991 เป็นบริษัทที่มุ่งเน้นในการสร้างและขายเครื่องมือวิทยาศาสตร์ มีการรับรองเช่น ISO9001, ISO14001, OHSAS18001 และ CE บริษัทผลิตเครื่องมือที่ทันสมัย รวมถึงซีรีส์ A3 สําหรับการค้นหาโลหะปริมาณเล็ก ๆ มันให้ ช่วยเหลือทั่วโลก และมุ่งเน้นคุณภาพและความคิดใหม่
รุ่นเช่น A3F และ A3G ออกแบบมาเพื่อการตรวจจับโลหะที่น่าเชื่อถือ
ที่ A3F (เปลวไฟ) และ A3G (เตากราไฟต์) รุ่นที่ทำเพื่อหาแมงกานีสอย่างแม่นยำในระดับที่แตกต่างกัน พวกเขามีเครื่องเผาโค้ด ซึ่งทําให้มันปลอดภัยและน่าเชื่อถือได้มากขึ้น

สรุปและสิ่งที่สำคัญ
การค้นหาและกำจัดแมงกานีสจากน้ำพื้นผิว เป็นส่วนใหญ่ของการจัดการระบบน้ำโรงงาน สเปคโตรโฟโตเมตรีการดูดซึมอะตอม เป็นเครื่องมือที่น่าเชื่อถือได้มาก ด้วยการตั้งค่าและมาตรฐานที่ถูกต้อง มันสามารถจับแมงกานีสได้ในระดับเล็กน้อย ลงถึงส่วนต่อพันล้าน
คำถามที่พบบ่อย
Q1: การดูดซึมอะตอม spectrophotometry มีความแม่นยำแค่ไหนเมื่อทดสอบความเข้มข้นต่ำของแมงกานีส?
ตอบ: สเปคโตรโฟโตเมตรีการดูดซึมอะตอมมีความแม่นยำมากในระดับต่ำ สามารถทำซ้ำผลได้ภายในประมาณ ± 0.02 mg L ⁻ ¹เมื่อใช้ขั้นตอนการตั้งค่าที่เหมาะสม
ไตรมาสที่ 2: สามารถใช้ AAS เปลวไฟได้หากน้ำพื้นผิวมีระดับแมงกานีสต่ำมาก (< 1 มก. ลิตร) ⁻¹)?
ตอบ: Flame AAS ทํางานด้วยการขยายขนาด 10x สําหรับระดับต่ำ แต่เตากราไฟต์ AAS ดีกว่าสําหรับระดับต่ำกว่า 1 มก. ลิตร ⁻¹. สามารถจุดได้ประมาณ 0.01 มก. ลิตร ⁻ ¹ ทำให้มันอ่อนโยนมาก
Q3: อะไรทําให้ซีรีส์ A3 ของ PERSEE เหมาะสำหรับการใช้งานในอุตสาหกรรม?
ตอบ: ซีรีส์ A3 มีเสถียรภาพใช้งานง่ายและมีซอฟต์แวร์ที่ฉลาด นี่ทําให้มันดีสําหรับแรงงานที่มีระดับทักษะที่แตกต่างกัน และสําหรับการใช้งานไม่หยุดในโรงงาน