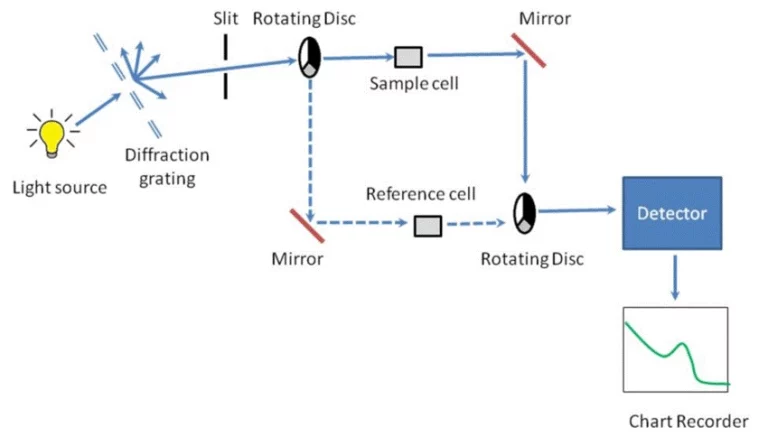
Spectrophotometry holds a key position in maintaining pharmaceutical quality. It allows for quick, non-harmful, and very exact checks of compounds at every stage of the drug process. This light-based method is vital in pharmaceutical labs. It helps confirm raw materials and ensures the final products stay consistent. It also meets all required rules.
One of the top companies worldwide that supplies solid tools for pharmaceutical spectrophotometry is เพอร์ส. The firm began with scientific advances back in 1991. It has strong skills in development and a strong focus on quality. PERSEE provides tools for light analysis that fit the tough needs of pharmaceuticals. Its range includes UV-VIS, FTIR, GC-MS, and others. These are made to match worldwide rules and lab processes.
How Is Spectrophotometry Used to Authenticate Raw Materials?
Keeping raw materials pure is a must in making drugs. Spectrophotometry gives a fast and accurate way to spot APIs. It does this through its special light absorption patterns. Moreover, the amount of a substance in a liquid can be found by looking at how much light it absorbs or lets through at different wavelengths. This check stops the use of wrong or low-quality starting items. As a result, it avoids problems down the line.
UV-Vis spectrophotometry works well for finding unwanted bits or broken-down parts early on. This happens in the supply chain. Spectrophotometers can check visible light or ultraviolet light. They go down to around 190nm in wavelength. Fast checks cut the chance of wasting money on bad batches. They also make sure rules are followed right from the start of making things.
How Does Spectrophotometry Ensure Uniform Drug Formulations?
Keeping drug mixes the same from one batch to the next matters a lot for safety. Spectrophotometry helps here. It does so by allowing exact number-based tests of APIs and fillers. Therefore, this means every pill, capsule, or liquid gives the right amount of drug. It supports steady results across production.
Methods that look at full light absorption patterns add more safety. They compare these to proven standard patterns. When linked to data tools, spectrophotometers help follow drug rule books. They do this by keeping track of how right the measures are over time. Such steps build trust in the process.
Can Spectrophotometry Predict Drug Stability?
Testing how long drugs last means putting them under stresses like heat, wetness, and light. Spectrophotometric methods can spot small changes in chemicals as time goes on. This helps set when products expire. By checking broken-down parts at set wave lengths, experts can build models of how things break down. These models use numbers. Not only does this help pick shelf life, but it also guides choices for packing and storage. Overall, it makes products last longer and safer.
How Does It Support Cleaning Validation in Manufacturing?
In places that follow good manufacturing practices, cleaning checks are very important. Spectrophotometry lets workers find leftover bits on machines after cleaning. These bits could be active drugs or cleaning liquids. Just like other tools, they need regular tests and checks to some degree. The method uses no harm to the samples and gives quick results. This way, checks can happen often without stopping work. It keeps things in line with rules from the FDA and EMA.
What Are the Key Spectrophotometric Techniques Used in Pharma QC?

UV-Vis Spectrophotometry: People use this a lot for daily quality checks. It is quick and easy to handle. It works with liquids and solids. It helps with number-based tests and gives steady results time after time. Tools such as the T7 UV-Vis spectrophotometer have clear optics that fit rule-based settings well.
FTIR Spectroscopy: This suits spotting group functions and checking tricky molecule setups. It gives detailed spectra for hard mixes. Uses include finding different forms of drugs and measuring water levels. These matter for solid drug shapes. Thus, it aids in understanding material properties deeply.
NIR Spectroscopy: This allows watching processes live, like pressing pills or mixing. Since it does not harm samples, it skips a lot of prep work. It speeds up choices right on the work floor. Workers can act fast based on real data.
What Instrument Features Matter Most in Pharmaceutical Applications?
Right wavelength matching makes sure light readings link well to molecule traits. Good calibration of wavelengths keeps checks steady across tests. Being able to repeat results is key to comparing batches. Photometric straightness allows exact counting over different amounts. Strong straightness means right counting across a broad range of strengths. Spotting tiny bits or broken parts needs a high feel for changes.
Blocking unwanted light boosts rightness at small absorption levels. Cutting stray light betters the spectrum truth, above all when absorption is low. Steady baselines make sure long watches during change studies are right. Sample ready work changes by type. Liquids often use small holders, while solids might need add-ons like surface touch units or spread light parts. All these features work together to make tools reliable for daily use in labs.
How Does Spectrophotometry Satisfy Regulatory Standards?
Big drug rule books like USP, EP, and JP require proof of how well spectrophotometers work. This covers straightness, wavelength rightness, and blocking stray light. Each rule book asks users to show their tools work right on points like straightness, wavelength, bandwidth, and stray light. Check plans must prove rightness, exactness, special focus, straightness, range, lowest detect levels, and strength under changing setups. Linking back to proven standard items adds more trust to the results. In this way, labs stay safe and approved worldwide.
Is Spectrophotometry Integrated into Pharma Manufacturing?
Today’s making of drugs uses both live (on-the-spot) and lab-based light checks. Live systems help check if mixes are even and when granulation ends. They do this without needing hand samples. Lab setups are common for testing batches before release. Both types fit into smooth workflows. Systems for handling data meet rules like 21 CFR Part 11. They store info safely with change logs. Linking to Lab Info Management Systems helps with easy record-keeping. This makes checks for rule watchers simpler.
Why Is Spectrophotometry Preferable Over Other Analytical Techniques?
When compared to methods like HPLC or GC-MS, spectrophotometry runs quicker and is easier to use. This proves helpful in early drug making or when testing many items. It uses fewer chemicals and keeps samples whole for more tests. That is key when samples are few or many checks are needed. As such, it fits well into busy lab routines without high costs or waste.
Why Choose PERSEE Instruments for Pharmaceutical Spectrophotometry?
Labs looking for steady tools with new ideas and rule matches will find PERSEE a solid choice. บริษัท has over thirty years of know-how. It offers a wide set of products. This includes the strong m7 quadrupole gc-ms เดี่ยว. PERSEE handles the main pharma tasks. These range from cleaning checks to testing leftover solvents. The M7 MS could be widely used in food safety, environmental protection, the material and chemical industry, life science, medicine research, criminal investigation, and many other fields. Beyond that, its tools have clear optics, tough software, and far-off fix options. These aid quick setup and long-term good work in places that follow good practices.
คำถามที่พบบ่อย
Q1: Can UV-Vis spectrophotometry replace HPLC in pharmaceutical quality control?
A1: UV-Vis gives quicker outcomes with easier sample ready than HPLC. However, it does not split compounds apart. It works best for regular tests. Still, it should pair with split methods when dealing with mixed items. This combo covers more ground effectively.
Q2: How sensitive are modern spectrophotometers in detecting impurities?
A2: Top tools show great feel for finding small unwanted parts. This holds when set against proven standards. Feel changes based on light setup and wave range. In practice, they catch issues that older ones might miss, aiding finer quality work.
Q3: How frequently should spectrophotometers be calibrated in a GMP environment?
A3: Experts suggest daily quick checks. Then, full setups come at set times based on how much the tool is used. This follows maker advice and drug rules like USP 857. Such steps keep everything running true and safe over time. Regular care ensures no surprises in results.