1. Resumen del método
Después de tratar la muestra, los iones plomo forman un complejo con dietilditiocarbamato de sodio (DDTC) bajo una cierta condición de pH. El complejo se extrae y se separa con 4-metil-2-pentanona (MIBK), a continuación se introduce en un espectrómetro de absorción atómica. Después de la atomización por llama, la absorbancia se mide a una longitud de onda de 283,3 nm. Dentro de un cierto intervalo de concentración, el valor de absorbancia del plomo es proporcional a su contenido, y la cuantificación se realiza mediante comparación con una serie estándar.
2. Instrumentos y reactivos
2.1 Instrumentos y equipos
2.1.1 Instrumentos de ensayo
| Número de serie. | Nombre | Cantidad | Requisitos técnicos | Accesorios |
|---|---|---|---|---|
| 1 | Espectrofotómetro de absorción atómica de llama | 1 conjunto | ...... 8211; | Lámpara catódica hueca de plomo |
| 2 | Compresor de aire | 1 conjunto | Presión nominal de descarga: 0,3 MPa | ...... 8211; |
| 3 | Acetileno Gas | 1 cilindro | Pureza ≥ 99,99% | ...... 8211; |
2.1.2 Equipo de pretratamiento de muestras
| Número de serie. | Nombre | Cantidad | Requisitos técnicos | Accesorios |
|---|---|---|---|---|
| 1 | Balanza electrónica | 1 conjunto | Sensibilidad: 0,1 mg | ...... 8211; |
| 2 | vaso | Varios | Volumen: 150 ml | ...... 8211; |
| 3 | Placa caliente ajustable | 1 conjunto | Rango de temperatura: Temperatura ambiente ~ 300 ℃ | ...... 8211; |
| 4 | embudo separador | Varios | Volumen: 250 ml | ...... 8211; |
| 5 | Micropipeta | 1 cada uno | Rangos: 100 μL ~ 1000 μL, 1000 μL ~ 5000 μL | ...... 8211; |
| 6 | Tubo colorimétrico | Varios | Volumen: 10 ml | ...... 8211; |
2.2 Reagentes
2.2.1 Reagentes en bruto
| Número de serie. | Nombre | Requisitos técnicos | Observaciones |
|---|---|---|---|
| 1 | 4-metil-2-pentanona (MIBK) | Reagente analítico (AR) | ...... 8211; |
| 2 | Amoniaco Agua | Reagente analítico (AR) | ...... 8211; |
| 3 | sulfato de amonio | Reagente garantizado (GR) | ...... 8211; |
| 4 | Citrato de amonio | Reagente analítico (AR) | ...... 8211; |
| 5 | Dietilditiocarbamato de sodio (DDTC) | Reagente garantizado (GR) | ...... 8211; |
| 6 | Bromothymol Azul | Reagente analítico (AR) | ...... 8211; |
| 7 | Ácido nítrico | Grado MOS | ...... 8211; |
2.2.2 Reagentes preparados
| Número de serie. | Nombre | Método de preparación | Observaciones |
|---|---|---|---|
| 1 | Solución acuática de amoníaco (1 1) | Medir 50 ml de agua de amoníaco, añadirlo a 50 ml de agua, y mezclar bien. | ...... 8211; |
| 2 | Solución de sulfato de amonio (300 g/L) | Pesar 30 g de sulfato de amonio, disolverlo en agua, y diluir a 100 ml con agua. | ...... 8211; |
| 3 | Solución de citrato de amonio (250 g/L) | Pesar 25 g de citrato de amonio, disolverlo en agua y diluir hasta 100 ml con agua. | ...... 8211; |
| 4 | Solución de dietilditiocarbamato de sodio (DDTC) (50 g/L) | Pesar 5 g de dietilditiocarbamato de sodio, disolverlo en agua y diluir hasta 100 ml con agua. | ...... 8211; |
| 5 | Solución Indicadora Azul de Bromotimol (1 g/L) | Pese 0,1 g de azul de bromotimol, disuelve en agua y diluya hasta 100 ml con agua, luego mezcle bien. | ...... 8211; |
| 6 | Solución de ácido nítrico (5 95) | Medir 50 ml de ácido nítrico, añadirlo a 950 ml de agua, y mezclar bien. | ...... 8211; |
2.3 Normas de referencia
2.3.1 Solución de stock
| Número de serie. | ¡No! No! | Nombre | Requisitos técnicos | Observaciones |
|---|---|---|---|---|
| 1 | GSB G 62071-90 | Solución estándar de plomo | Concentración: 1000 μg/ml | Instituto Central de Investigación de Hierro y Acero, Centro Nacional de Pruebas de Materiales de Hierro y Acero |
3. Cálculo de resultados
El contenido de plomo se expresa como fracción de masa El W de plomo (Pb), en unidades de mg/kg, calculado mediante la siguiente fórmula:
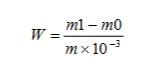
Dónde:
- M1= Masa de plomo en la solución de ensayo obtenida a partir de la curva de trabajo, unidad: miligramos (mg);
- M0 = Masa de plomo en la solución de ensayo en blanco obtenida a partir de la curva de trabajo, unidad: miligramos (mg);
- metro = Masa de la muestra, unidad: gramo (g).
La media aritmética de los resultados de determinación paralela se toma como resultado de determinación final. La diferencia absoluta entre los dos resultados de la determinación paralela no excederá de 1 mg/kg.

