1. Aperçu de la méthode
Après le traitement de l'échantillon, les ions plomb forment un complexe avec le diéthyldithiocarbamate de sodium (DDTC) sous une certaine condition de pH. Le complexe est extrait et séparé par la 4-méthyl-2-pentanone (MIBK), puis introduit dans un spectromètre d'absorption atomique. Après atomisation de flamme, l'absorbance est mesurée à une longueur d'onde de 283,3 nm. Dans une certaine gamme de concentration, la valeur d'absorbance du plomb est proportionnelle à sa teneur, et la quantification est effectuée par comparaison avec une série standard.
2. Instruments et réactifs
2.1 Instruments et équipements
2.1.1 Instruments d'essai
| N° de série. | Nom | Quantité | Exigences techniques | Accessoires |
|---|---|---|---|---|
| 1 | Spectrophotomètre d'absorption atomique de flamme | 1 ensemble | & quot; 8211; | Lampe cathodique creuse au plomb |
| 2 | Compresseur d'air | 1 ensemble | Pression nominale de décharge: 0,3 MPa | & quot; 8211; |
| 3 | gaz acétylène | 1 cylindre | Pureté ≥ 99,99% | & quot; 8211; |
2.1.2 Équipement de prétraitement des échantillons
| N° de série. | Nom | Quantité | Exigences techniques | Accessoires |
|---|---|---|---|---|
| 1 | Équilibre électronique | 1 ensemble | Sensibilité : 0,1 mg | & quot; 8211; |
| 2 | Bécher | Plusieurs | Volume: 150 mL | & quot; 8211; |
| 3 | Plaque chaude réglable | 1 ensemble | Plage de température: Température ambiante ~ 300 ℃ | & quot; 8211; |
| 4 | entonnoir séparateur | Plusieurs | Volume: 250 mL | & quot; 8211; |
| 5 | Micropipette | 1 chacun | Gammes: 100 μL ~ 1000 μL, 1000 μL ~ 5000 μL | & quot; 8211; |
| 6 | Tube colorimétrique | Plusieurs | Volume: 10 mL | & quot; 8211; |
2.2 Réactifs
2.2.1 Réactifs bruts
| N° de série. | Nom | Exigences techniques | Remarques |
|---|---|---|---|
| 1 | 4-méthyl-2-pentanone (MIBK) | Réactif analytique (AR) | & quot; 8211; |
| 2 | Ammoniaque Eau | Réactif analytique (AR) | & quot; 8211; |
| 3 | sulfate d'ammonium | Réactif garanti (GR) | & quot; 8211; |
| 4 | Citrate d'ammonium | Réactif analytique (AR) | & quot; 8211; |
| 5 | Diéthyldiithiocarbamate de sodium (DDTC) | Réactif garanti (GR) | & quot; 8211; |
| 6 | Bromothymol Bleu | Réactif analytique (AR) | & quot; 8211; |
| 7 | Acide nitrique | Classe MOS | & quot; 8211; |
2.2.2 Réactifs préparés
| N° de série. | Nom | Méthode de préparation | Remarques |
|---|---|---|---|
| 1 | Solution aqueuse d'ammoniac (1 1) | Mesurer 50 mL d'eau d'ammoniac, l'ajouter à 50 mL d'eau et bien mélanger. | & quot; 8211; |
| 2 | Solution de sulfate d'ammonium (300 g/L) | Peser 30 g de sulfate d'ammonium, le dissoudre dans l'eau et diluer à 100 mL avec de l'eau. | & quot; 8211; |
| 3 | Solution de citrate d'ammonium (250 g/L) | Peser 25 g de citrate d'ammonium, le dissoudre dans l'eau et diluer à 100 mL avec de l'eau. | & quot; 8211; |
| 4 | Solution de diéthyldiithiocarbamate de sodium (DDTC) (50 g/L) | Peser 5 g de diéthyldithiocarbamate de sodium, le dissoudre dans l'eau et diluer à 100 mL avec de l'eau. | & quot; 8211; |
| 5 | Solution d'indicateur bleu de bromothymol (1 g/L) | Peser 0,1 g de bromothymol bleu, le dissoudre dans l'eau, diluer à 100 mL avec de l'eau, puis bien mélanger. | & quot; 8211; |
| 6 | Solution d'acide nitrique (5 95) | Mesurer 50 mL d'acide nitrique, ajouter à 950 mL d'eau et bien mélanger. | & quot; 8211; |
2.3 Normes de référence
2.3.1 Solution de stock
| N° de série. | - Non, non. | Nom | Exigences techniques | Remarques |
|---|---|---|---|---|
| 1 | GSB G 62071-90 | Solution standard de plomb | Concentration: 1000 μg/mL | Institut central de recherche sur le fer et l'acier, Centre national d'essai des matériaux en fer et en acier |
3. Calcul des résultats
La teneur en plomb est exprimée en fraction de masse W de plomb (Pb), en unités de mg/kg, calculé selon la formule suivante:
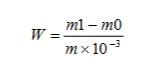
Où:
- M1= Masse de plomb dans la solution d'essai obtenue à partir de la courbe de travail, unité: milligramme (mg);
- m0 = Masse de plomb dans la solution blanche obtenue à partir de la courbe de travail, unité: milligramme (mg);
- m = Masse de l'échantillon, unité: gramme (g).
La moyenne arithmétique des résultats de la détermination parallèle est prise comme résultat final de la détermination. La différence absolue entre les deux résultats de détermination parallèles ne doit pas dépasser 1 mg/kg.

