Infrared Spectroscopy is a helpful tool for scientists, students, and workers who want to learn about the makeup and shape of particles. Whether you’re studying chemistry, working on medicines, or checking the environment, an infrared spectrometer gives important details about particle features. In this blog, we explain the basics, parts, ways, uses, and limits of Infrared Spectroscopy. At PERSEE, we are excited to support your work with new tools made just for you.
Fundamentals of Infrared Spectroscopy
Infrared Spectroscopy is a method that studies how particles take in infrared light to show their shape and makeup. It’s used a lot in science because it’s accurate and flexible.
The Principles of Infrared Absorption
Infrared Spectroscopy passes infrared light through a sample. Particles take in certain light waves. This makes their bonds shake. These shakes create a special pattern, like a particle’s unique mark. Scientists use this pattern to find out what’s in the sample. The light waves match the energy needed to make bonds move, usually in the infrared range (4000–400 cm⁻¹).
The Role of Molecular Vibrations in IR Spectroscopy
Particle shakes are the key to Infrared Spectroscopy. When infrared light hits a particle, it starts movements like stretching or bending of bonds. Each bond type (e.g., C-H, O-H, C=O) shakes at its own speed. This makes clear peaks in the pattern. By studying these peaks, you can find out about particle shapes with great accuracy.
The Interaction Between Infrared Radiation and Molecules
Infrared light and particles work together based on the particle’s electric balance. Only bonds that change their balance during shaking take in infrared light. For example, balanced particles like O₂ or N₂ don’t show up in IR. But uneven bonds like C=O or N-H make strong signals. This makes Infrared Spectroscopy great for studying complex particles.
Components of an Infrared Spectrometer
An infrared spectrometer is a smart tool built to measure infrared light taken in by samples. Its main parts ensure steady and clear results.
Key Features of an Infrared Spectrometer
A typical infrared spectrometer has:
- Light Source: Sends out wide infrared light.
- Sample Area: Holds the sample (solid, liquid, or gas) for testing.
- Wavelength Selector: Picks or adjusts certain light waves.
- Detector: Checks the strength of light that passes through.
- Data System: Shows and processes the pattern.
These parts work together to give correct patterns. Our T60Vspectrometer is made with top features for high sensitivity and quick results.
Types of Infrared Spectrometers
Infrared spectrometers come in two main kinds, each good for different tasks.
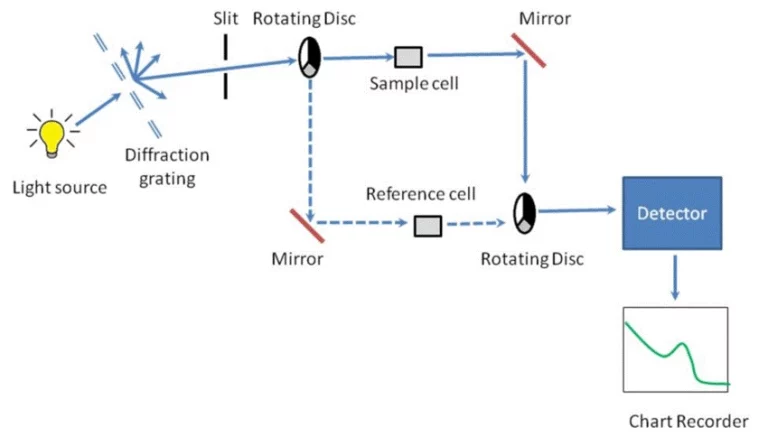
Dispersive Infrared Spectrometers
Dispersive infrared spectrometers use a prism or grating to split infrared light into single waves. They are steady for simple tasks but work slowly. They’re less sensitive than newer options. These are best for basic tests where high clarity isn’t needed.
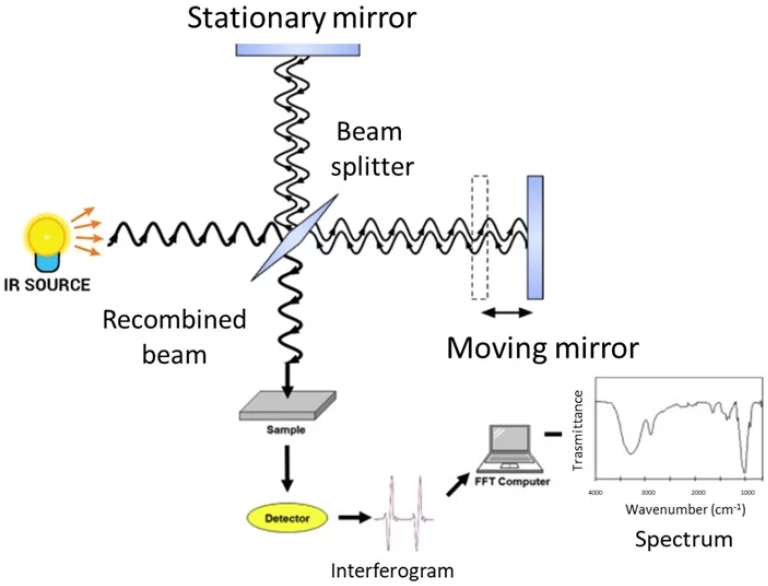
Fourier Transform Infrared (FTIR) Spectrometers
FTIR spectrometers use a special device to collect all light waves at once. This makes tests faster and clearer. They have better signal strength. FTIR is the top choice for tricky tests in research and industry. Our TU400-VIS FTIR spectrometer gives quick scans and great accuracy for tough tasks.
Methods for Analyzing Molecular Structures Using IR Spectroscopy
Infrared Spectroscopy offers many ways to study particle shapes, making it a useful tool for researchers.
Identifying Functional Groups Through IR Absorption Peaks
Each group in a particle (e.g., hydroxyl, carbonyl, amine) takes in infrared light at certain waves. This makes special peaks. For example:
- C=O (Carbonyl): Takes in around 1700 cm⁻¹.
- O-H (Hydroxyl): Takes in around 3200–3600 cm⁻¹.
- C-H (Alkane): Takes in around 2800–3000 cm⁻¹.
By matching peaks to known patterns, you can find groups in unknown samples easily.
Determining Bond Strengths and Molecular Interactions
Infrared Spectroscopy shows bond strength by checking the energy needed for shakes. Strong bonds, like triple bonds, take in at higher speeds than weak bonds, like single bonds. It also finds particle connections, like hydrogen bonds, which shift peaks. This helps you understand particle stability and reactions.
Differentiating Isomers with IR Spectral Patterns
Isomers have the same particle formula but different shapes. They make unique IR patterns. For example, isomers of ring compounds show special peak shapes due to bond differences. This makes Infrared Spectroscopy helpful for telling isomers apart in chemistry and medicine.
Applications of Infrared Spectroscopy in Various Fields
Infrared Spectroscopy is used in many areas, giving key details for research and quality checks.
Use in Organic Chemistry for Structural Elucidation
In chemistry, Infrared Spectroscopy finds groups and confirms particle shapes. It’s vital for making new compounds and checking reaction results. Scientists use IR patterns to ensure bonds form correctly.
Role in Pharmaceutical Analysis and Drug Development
In medicines, Infrared Spectroscopy checks drug purity and watches production steps. It finds impurities, confirms active parts, and tests drug mixes. FTIR spectrometers, like our TU400-VIS, give the accuracy needed for rules and standards.
Application in Environmental Monitoring and Material Science
In environmental work, Infrared Spectroscopy checks harmful substances like gases in air or water. In material science, it studies plastics and mixed materials, ensuring quality and strength. Its flexibility makes it essential for these fields.
Advantages and Limitations of Infrared Spectroscopy
Infrared Spectroscopy has special benefits but also some challenges to know about.
Benefits of Using IR Spectroscopy for Molecular Analysis
Infrared Spectroscopy is valued for its:
Non-Destructive Testing Capabilities
IR tests samples without breaking or changing them. This is great for rare or limited samples, like biological tissues or special chemicals.
High Sensitivity to Functional Groups
The method easily spots specific groups, ensuring correct identification, even in mixed samples.
Challenges and Constraints in IR Spectroscopic Analysis
Infrared Spectroscopy has some limits:
- Sample Prep: Some samples need careful setup to avoid issues from water or other substances.
- Limited Number Checks: IR isn’t great for exact counting compared to methods like UV-Vis.
- Mixed Samples: Overlapping peaks in mixtures can make results hard to read without special tools.
PERSEE: A Trusted Supplier of Infrared Spectrometers
At PERSEE, we are proud to lead in analytical tools since 1991. Our skill and focus on quality make us a dependable partner for your lab needs.
Overview of PERSEE’s Product Offerings in IR Technology
We offer infrared spectrometers built for accuracy and ease. Our T60V and TU400-VIS spectrometers give fast, correct results for tasks from research to quality checks. They have clear optics, easy controls, and work with many sample types.
Why Choose PERSEE for Your Analytical Needs
Our products meet standards like ISO9001, ISO14001, OHSAS18001, and CE, ensuring trust and quality. We offer global support through our service team, keeping your tools working well. Visit our homepage to see our full tools or contact us for personal help.
Conclusion: The Importance of Infrared Spectroscopy in Modern Science
Infrared Spectroscopy is a key part of modern science, giving clear particle analysis across fields. From finding groups to checking drug quality, its uses are wide. At PERSEE, we are dedicated to helping you with new infrared spectrometers. Check our products and let us support your goals.
Frequently Asked Questions (FAQs)
Q1 What is the primary purpose of infrared spectroscopy?
A1 The main goal of Infrared Spectroscopy is to find and study particle shapes by checking how they take in infrared light. It shows groups, bond types, and particle connections, making it vital for chemical analysis.
Q2 How does an FTIR spectrometer differ from a dispersive spectrometer?
A2 An FTIR spectrometer uses a device to collect all light waves at once. This makes it faster and clearer. A dispersive spectrometer splits waves one by one, so it’s slower and less sensitive. It’s good for simpler tests.
Q3 Can infrared spectroscopy be used to analyze mixtures?
A3 Yes, Infrared Spectroscopy can test mixtures by finding groups through special peaks. But overlapping patterns may need advanced tools or separation methods for clear results.


113-1.webp)