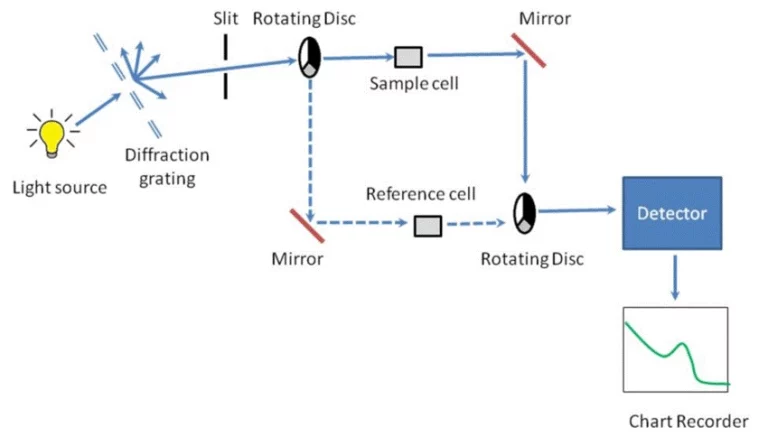
정확한 농도 측정은 종종 기술적 인 도전처럼 느특특히 샘플이 미세하거나, 빛에 민감하거나 신뢰할 수 있는 반복성이 필요할 때.분광광계는 샘플이 얼마나 많은 빛을 흡수하는지 직접 사용할 수 있는 숫자 결과로 변환함으로써 이것을 해결합니다.과학적 측면에 들어가기 전에, 신뢰할 수 있는 기기가 방법만큼 중요한다는 것을 알고 있도록 도와줍니다.오늘날 많은 연구소는 안정적인 광학 시스템, 실용적인 소프트웨어 및 일상적인 화학, 환경 및 교육 사용을 위해 제작된 장치를 위해 PERSEE를 사용합니다.

분광광계가 농도를 정확하게 측정할 수 있는 원칙은 무엇입니까?
분광광계는 샘플을 통해 빛을 지향하고 그 빛이 얼마나 흡수되는지 감지함으로써 작동합니다.흡수된 양은 농도와 직접 연결됩니다.이 원리는 예측 가능하게 행동하기 때문에 수십 년 동안 실험실 분석의 이 이 이 원리가 되었습니다.안정적인 광학으로 구축된 시스템을 사용할 때 연산자가 변경되더라도 숫자는 매일 일관되어 있습니다.
정밀 광학을 통한 파장 선택
측정은 다른 파장에서 가능하지만 흡수 최대를 선택하면 감도, 정밀도 및 선형 동적 범위가 향상됩니다.나쁜 파장 정확도는 드리프팅 결과를 초래합니다.기기와 같은 TU400 VIS 안정적인 광학 부품을 사용하여 파장 정렬을 안정적으로 유지합니다.같은 테스트의 여러 배치를 실행하거나 몇 주 후 이전 작업을 반복해야 할 때 이러한 안정성은 도움이 됩니다.

TU400 및 TU500의 신호 감지 및 교정 안정성
감지기 품질은 전체 워크플로우에 영향을 미칠 수 있습니다.탐지기가 안정적이고 소음이 낮으면 감감소한 농도의 변화가 배경에서 잃어버린 대신 보이게 됩니다.광범위한 분광 범위 (일반적으로 190-800 nm)는 유기 및 무기 종의 UV 전환과 색깔 복합체의 가시적 인 전환 모두에 액세스를 제공합니다. TU500 UV-VIS 광범위한 샘플 유형에 적용 가능합니다.
파장 정밀도 측정 신뢰성에 어떻게 영향을 미치는가?
1 nm 이동조차도 심심지어 심지심심지어 심심심지어 1 1 1 1 nm 이동조차도 심심심지어 심심지어 1 1 1 1 1 nm 이동조차도 심심지어 심심지1 nm 이동조차도 심심심지어 심심지라도 심심심지어 심심심심지염료, 금속 이온 또는 유기 화합물과 같은 샘플과 함께 작업하는 경우 흡수 정점은 흡흡수성 정점이 염염염료가 염염염료료, 금속 이온 또는 유기 화합물과 같은 샘플을 사용당신은 그 정상을 반복적으로 타기 위해 기기가 필요합니다.그렇지 않으면, 당신의 준비가 잘 될 때에도 결과는 무작위로 보일 수 있습니다.
TU500 UV-Vis의 고안정성 광학 부품의 역할
TU500은 안정적인 광학 벤치를 사용하여 파장이 긴 세션에서도 정렬하게 유지되도록 도와줍니다.
분석 정확도에 대한 스트레이 라이트 제어의 영향
스트레이 라이트는 광학 시스템을 통해 미끄러지고 검출기에 도달하는 빛입니다.t. 너무 많은 방황 빛은 흡수 곡선을 평평하게 하고 높은 흡수 수준에서 정확성을 낮추습니다.좋은 광학 디자인은 이를 줄여 측정을 완전한 동적 범위를 유지할 수 있습니다.
낮은 농도 샘플에 대한 분광 해상도의 중요성
스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스스이것은 작은 변화가 중요한 낮은 농도의 샘플을 사용할 때 도움이 됩니다.날카로운 해상도는 또한 날날날카로운 해상도는 날날날날카로운 해상도는 날날날날날카로운 해상상도도
왜 교정 정확한 정량에 필수적입니까?
캘리브레이션은 미래의 모든 판독에 대한 참조점처럼 작동합니다.적절한 교정 곡선으로 추측없이 새로운 샘플을 테스트 할 수 있습니다.샘플 매트릭스가 약간 변경되더라도 잘 준비된 곡선은 계기를 올바른 농도로 안내합니다.
재생가능한 광원을 사용하여 표준 곡선 설정
UV-Vis 계기는 UV 영역에 대한 UV 램프와 가시할 수 있는 영역에 대한 UV UV UV UV-Vis 램프를 사용하고 있으며, 특히 UV UV 램UV-Vis 계기에서의 강도 드리프트는 시간이 지남에 따라 교정 곡선을 변경 할 수 있습니다.광원이 안정적으로 유지되면 표준 곡선이 예기치 않게 변하지 않습니다.
PERSEE 계기의 자동화 교정 지원
PERSEE 제품 라인의 많은 계기에는 가이드 단계가 포함되어 있으며, 예측 가능한 순서로 예예비 및 교정을 통해 안내합니다.이것은 운영자 실수를 줄입니다.워크플로우는 분광학에 익숙하지 않은 사람에게도 간단합니다.
A3F 원자 흡수 시스템을 통해 인간 오류를 줄이기
일부 산업에서 금속을 미적 수준으로 정량화해야 할 수 있습니다.흡수량이 매우 낮으면 원자 흡수는 더 높은 선택성을 가져옵니다.A3F 모델은 램프 제어와 자동화된 기능의 조합을 사용하여 준비 오류를 최소화합니다.

샘플 처리와 쿠베트 품질은 결과에 어떻게 영향을 미칩니까?
최고의 기기조차도 최악한 샘플 처리를 보상할 수 없습니다.쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠쿠이러한 작은 단계에 관심을 기울이는 것은 보통 정확도를 눈에 보이게 향상시킵니다.
쿠베트 재료 및 경로 길이의 영향
유리, 석영 및 플라스틱 유유리는 모두 다르게 행동합니다.석영은 플라스틱이 그 범위에서 너무 강하게 흡수하기 때문에 UV 작업에 사용됩니다.표준 10 mm 경로 길이는 일반적이지만, 더 긴 경로는 매우 매매매우 표표표준 매우 표표준 표준 10 mm 경로는 매우 표표표준 표준 10 mm 경로 길이는 일반적입니다.
오염 및 지문 방해 최소화
지문이 예상치 못한 흡수 스파이크를 도입하는 것을 보는 것은 일반적입니다.가루 없는 피복으로 쿠쿠작은 조각조차도 낮은 수준의 측정을 변경할 수 있습니다.
온도 및 혼합 균일성 관리
온도는 용매 구절 지수를 변경하고, 스스스온도 대역을 확대하고, 화학 균형을 변경함으로써 흡수성에 영향을 미치므로 샘플은 일관된 측정을 위해 열정화되어야합니다.샘플을 실온에서 유지하고 측정 전에 적절하게 혼합하면 갑자기 변화를 피할 수 있습니다.
측정 정밀도에서 감지기 감도가 어떤 역할을하는가?
감지기는 빛을 전기 신호로 변환합니다.민감한 감지기는 작은 변화를 쉽게 구별할 수 있습니다.이것은 환경 테스트 또는 약제 분석에서 결과가 종종 하단 한계에 가까이 떨어지는 데 중요합니다.
Photodiode 및 PMT 성능 차이점
PMT는 높은 감도를 요구하는 단색 시스템 스캔에 사용되지만 다이오드 배열 계기는 CCD 또는 CMOS 탐지기를 사용하여 빠른 전체 스전전전트럼 수집을 위해 사용합니다.선택은 샘플 유형과 가장 자주 작업하는 농도 범위에 따라 달라집니다.
TU400 Vis의 소음 감소 기술
TU400은 소음 감소 회로 설계를 사용하여 깨끗한 기본선을 유지하는 데 도움이 됩니다.깨끗한 기본선은 전기 변화 뒤에 숨어있는 차이를 볼 수 있기 때문에 중요합니다.
높은 및 낮은 흡수 범위에서 선형 반응을 유지
탐지기가 반응을 선형으로 유지할 때, 농도를 두 배로 늘리면 흡수량을 두 배로 늘릴 것이라고 신뢰할 수 있습니다.비선형 탐지기는 교정 곡선을 예측할 수 없게 만듭니다.
현대 분광광계는 반복성과 안정성을 어떻게 지원합니까?
반복성은 모든 분석 기기에 대한 가장 중요한 지표 중 하나입니다.두 개의 연산자가 같은 테스트를 반복하면 거의 같은 숫자를 얻어야합니다.이것은 프로세스에 대한 신뢰를 구축하고 불필요한 재테스트를 피하는 데 도움이 됩니다.
내부 참조 시스템 및 장기 드리프트 제어
많은 UV-Vis 계기는 기본선을 안정시키고 램프 드리프트를 수정하기 위해 이중 많은 광많은 광많은 광학 디자인 또는 참조 보상을 사용합니다.이것은 특히 장기 모니터링 작업 중에 기준선을 안정적으로 유지합니다.
자동화된 자기 진단 및 기본선 수정
자기 진단 검사는 무언가에 관심이 필요할 때 경고합니다.기본선 수정은 램프 온난화 또는 파장 변경 후에도 장치를 준비 할 수 있습니다.
TU 시리즈의 기계적 및 광학적 안정성
견고한 디자인은 진동을 감소시키며 광학과 전자 장치를 모두 보호합니다.이것은 작은 것처럼 보일 수도 있지만 안정적인 구조는 종종 더 적은 재校准으로 이어집니다.
왜 PERSEE 계기는 정확한 농도 분석을 위한 강력한 선택입니까?
신뢰할 수 있는 흡수 데이터를 지원하는 견고한 광학 설계
안정적인 광학 시스템은 배치에 걸쳐 측정을 일관적으로 유지합니다.몇 주 후 테스트를 반복하거나 다른 운영자들 사이에 결과를 공유해야 할 때 이것은 중요합니다.
여러 분석 모드를 지원하는 사용자 친화적 인 소프트웨어
명확한 메뉴는 워크플로우를 더 빠르게 만듭니다.연산자는 인터페이스를 다시 배우지 않고 표준 곡선, 동학 또는 파장 스캔 사이를 전환할 수 있습니다.
TU400/TU500에서 A3F 시스템까지 광범위한 응용 범위
가시적 범위 테스트가 필요하든, 전체 UV 비스 적용 또는 미적 금속에 대한 원자 흡수, 제품 범위는 샘플 유형과 일치하는 여러 가지 선택권을 제공합니다.
FAQ는
Q1: 왜 흡수 판독은 때로는 시간에 따라 드리프트합니까?
A: 드리프트는 온도 변화, 램프 노화 또는 파장 불안정성에서 발생할 수 있습니다.기기를 따뜻하게 하고 기기기를 청소하는 것은 종종 도움이 됩니다.
Q2: 다른 cuvette 재료는 흡수 변경합니까?
A: 예.석영은 UV 빛을 더 잘 전달하기 때문에 UV에 사용됩니다.플라스틱은 대부분의 UV를 차단하여 결과에 영향을 미치게 됩니다.
Q3: 교정 곡선을 얼마나 자주 재구축해야 합니까?
A: 시약이 변경될 때, 램프가 나이가 들 때 또는 결과가 예상치 못한 변화를 보여주기 시작할 때 다시 구축해야합니다.