荧光分光学は強力な分析ツールです。それ’科学研究室、工場、病院で一般的なs。この方法は、エネルギーを吸収した後に材料から放出される光を見ています。その物質について明確な詳細を提供します。しかし“荧光”たくさんの土地を覆う。この技術は非常に異なる2つのサイズで動作します 小さな分子規模とさらに小さな原子規模です
このガイドはまず主なアイデアを分解します。その後、それを使用するための3つの大きな方法に潜みます。 There’s分子s分分子sss分子ssss分子sssss分子複雑な分子をチェックするための通常の選択。次に、元素を見つけるための難しいツールであるX射線次次次次に、X射線次次に、X射線次次に、X射線次次次に、X射線次次次に、元素を見つけるための難しいツールである。そして最後に、特定の有害な元素を見つける超敏感な方法である原子原原原原子原原原原原原原子最最最後に、原子最最最後に、原子原原原原原子原原原原原原子最最後に、原それぞれの走行方法を知ることは、仕事に最適なものを選ぶための鍵です。
荧光の原理
物質はエネルギーを吸収し、光を吐き出します。電子はより高い場所に飛び込む。その後、戻って落ちます。その過程で、光子を射出します。その’S The Glowこれらの方法を区別するのは何ですか。それ’激起されるもの――分子全体、あるいは単一の原子。さらに、物事を起動させるために使用されるエネルギーの種類です。
1. 分子1. 1. 分子分分子1 1 1. 分子1 1 1. 分子1 1 1 1. 分子1 1 1 1. 分子1 1 1 1. 分子1 1 1 1. 分子1 1 1 1 1. 分子1 1
これは、人々が“について話すときに行くための技術です。”それ’生物学、材料作業、健康研究の大きな問題です。
分子レベルでどのように機能するか
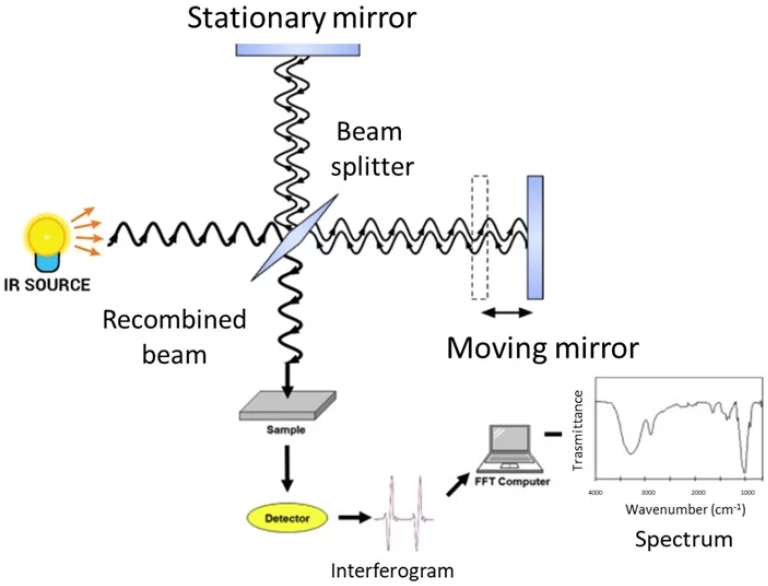
これを想像してみてくださいこの方法は、低エネルギーの紫外線または可視光(通常は200〜800ナノメートルの間)を捕捉します。それは分子の外部の電子に衝突します。分子は冷たくなり、正常に戻ります。長波の光子を放出する。その’ストークズは行動に移動する。光のパターン?それ’s は分子とその周囲の個人的なタグのようです。ここの機器はスペクトロフローメーターですそれは光のためのクセノンランプ、波長を分類するフィルター、および光増殖チューブ(PMT)のような光光光増殖チューブのような光光光光光増殖チューブ(PMT)のような光光光光のシャープな検出器を搭載しています。
検出するもの
- 有機および生物分子植物からの染料、薬、および緑 ( GFP)のような体内の重要な兆候のような明るくなるものを 。
これらのものをすぐに発見します。敏感性?ナノモルレベルまたは10まで⁻⁹ M. That’S 小さい。
- 分子相互作用このツールは、分子の変化を感じます’S 世界。液体タイプ、酸レベル、または空気泡による光強度の変化。それは’タンパク質がタタンパク質がタタタンパク質がタタタンパク質がタタンパク質がタタンパク質がタタタタタンパク質がタタタタンパク質がタタタンパクWhat’さらに、ナノ秒からピコ秒までの速いダンスの量または時間ベースの安定したチェックを処理します。
まず、安定状態ままずまままず、安定状態ままず、安定状態ままず、平均的な光を捕捉して分子を数えるだけです。時間解決?光がどのように消えるかを追跡します。それは物の中の隠された動きを明らかにします。
2. 原子レベルの2 つの異なる技術
すぐギアをシフト。あなた’単一の原子(バンドルされた分子ではない)の後、アプローチは変わります。ここに2つの主要な道が出てきます。
2.1.X線X射線X射線XX射線XX射線XXX射線XXX射線XXXX射線XXXX射線XXX射線XXX射X射線X射X射X射
XRFはパンチをパックします。それ’ ’を見つけるために非有害で素晴らしい元素レベルでサンプルのs。
原子レベルでどのように機能するか
柔らかい光を忘れ。これは1から100 keVの強いX線を使用します。ビームは原子から放散した電子を爆破します’s コアシェル。では?外部の電子がそれを修正するためにスライドします。 ブーム - 新しいX線が“として出てきます。荧光”その ray’S パワー?それ’元素のための死んだギフェアです。
検出するもの
- 元素構成XRFはマグネシウム(Mg)やアルミニウム(Al)からウラン(U)まで基本的なものを迅速にスキャンします。それはppmの痕跡(百万分の一部)を捕捉します。その答えの大きな質問は、“What’これらのもののメイクアップは、要素的に?”
それに加えて、it’固体または固体のために便利なs。準備は必要ありません。
- アプリケーション: 316ステンレス鋼のバッチをチェックする金属店を考えてください。あるいは岩犬が地球層をマッピングしている。ウォッチドッグさえ 古いペンキで古古古い古古古いペンキに古古古いペンキの古古古いペンキにウウウォウウウォッチドッしたがって、品質チェック、地球科学、グリーンウォッチの努力に適しています。
2.2.原子原原子原原子原原原子原原原子原原原原子原原原原子原原原原子原原原原子原原原原原子原原原原原原子原原原原原
AFSは深くなります。それ’いくつかの要素のために挑発的で超Sharpです。
原子レベルでどのように機能するか
ステップ1:サンプルを分離します。もはや分子結合はなく、ガス雲の中の自由原子だけです。その’原子化。次に、空のカソードランプのような標的光は、正確な波を発射し、望ましい原子だけを目覚めます。答えに照らされる。検出器はその純検信号を捕捉します。設定?原子原子荧光分光計。
検出するもの
- 特定の微量有毒元素AFSは’Tはすべて。アセン(As)、水銀(Hg)、セレン(Se)、カドミウム(Cd)などの毒物の超低狩りで輝く。制限?ppbまたはpptさえ、ターゲットとミックスに基づいて。
それは’ほとんどの人にとってoverkill。しかし’ポイント - 厳しいルールを満たします。
- アプリケーション食品チェックはリストのトップです。FDA’の下で水銀のための魚をスキャンすることを考えてください。s 1.0 ppmの帽子。環境テストもあります。それは他のツールをSharpnessに打ち負かすので、コンプライアンスは簡単です。
概要と比較
| 特徴 | 分子荧光 | X線X射線X射線XXRF | 原子原子原子原原子原原原子原原原子原原原原子原原原子原原原原子原原原原子原原原原原子原原原原原原子原 |
|---|---|---|---|
| 主目標 | 分子 | 原子(広範囲) | 原子(特定数) |
| 刺激源 | 紫外線可視光 | X線 | 特定の波長光 |
| サンプル状態 | 通常は液体溶液 | 固体、粉末、または液体 | サンプルはガスに原子化されます |
| 情報 | 分子構造,浓度 | 元素構成 | 超微量元素浓度 |
| キーユーズケース | 生物学研究,薬学 | 品質管理、地質学 | 食品安全、環境テスト |
クイックノート:各行は分割を強調します。分子側は湿れ、動かしくなります。原子の?乾燥した事実やガス雲。
信頼できるメーカーからの機器:PERSEE
正しいギアを選ぶ トンが大切です。分子や原子のスケールに一致します。固体メーカーのような 忍耐 すべての基地をカバーします。
有毒な原子のそのそのそれそれそれらの毒毒毒性原子のそれそれそれらの毒毒毒毒性原子のそれそれそれらのそれそれそれらのそれそれらのそれそれそれそれらのそれそれそれそれそれらいいい 原子荧光分光計。 彼ら’食品とエコ規則のために厳しく調整された。ショートカットはない

結論
3つは全て3つの全て全全て全全全全3つは全全て全全てが全全て3つは33333つまり全全全て全て全全全てが全全全て33つまり全てしかし、彼らの仕事?夜と日パズルに基づいて選択します。纠缠した分子とそのチャットを解除したいですか?分子分子分分分子分分子分分分子分分分子分分子分子分子分子分分子分子分分 分子分分子分子分分分分子分未加工要素のラインナップが必要ですか?XRFルール有毒原子の痕跡を特定するために?AFSは手を下げる。
よくある質問:
Q1: Q1: Q1: Q1: Q1: Q1: QQQ1: QQQ1:QQ1: QQQ1: QQ1: QQ1 Q1: QQ1: Q1:
A: どちらもエネルギーのヒットと光のバックからキックオフします。しかし、しかししかししかししかししかししかし、しかししかししかししかししかししかししかししかししかししかししかししかししかししかししかししかししかししかししかししかししかししかししかししかし しかししかししかししかししかししかししかししかししかし電子の経路が遅くなると、グロースティックのように、数秒または数分間続く光を意味します。それ’スピード。ナノ秒のみ。
Q2:Q2:Q2:Q2:Q2:Q2:QQQ2:QQQQQQ2:QQ2:QQ2:QQ2:QQQ2:Q2
A: もちろんしかし、それはスタイルに依存します。XRFは金属原子をまっすぐに見つける。AFSは水銀やカドミウムのような特定のものを水水水水水水銀やカドミウムのように水水水銀やカドミウムのような特定のものをAFAFAFAFSは水水水水銀やカドミ分子荧光は直接ヒットをスキップします。代わりに、それはスマートなプローブ - その金属を捕まえるときにのみ輝く分子を使用します。
Q3:なぜサンプルはAFSのためのガスに変わるが、XRFのためではないのですか。
A: AFSはガスの形で自由原子を要求します。そのように、ランプ’光は分子の混乱なしで標的にしか打つ。XRF?これらのX線は固体や液体を簡単に穿透します。内部の電子は設定にかかわらず振動します。ガスは必要ありません。