1. 方法概要
サンプルを処理した後,サササンプルイオンは,特定のpH条件の下で,ディエチルディチオカルバメン酸ナトリウム (DDTC) と複合体を形成します.複合体を抽出し、4-メチル-2-ペンタノン(MIBK)で分離し、原子吸収分光計に導入する。炎の原子化後、吸収率は283.3nmの波長で測定されます。特定の浓度範囲内で,一一一定の一一定の浓度範囲内で,一一定量化は標準シリーズと比較して量化されます.
2. 器具および試薬
2.1 機器と機器
2.1.1 テスト機器
| シリアル番号 | 名前 | 量 | 技術要件 | アクセサリー |
|---|---|---|---|---|
| 1 | 火焰 原子 吸収 分光 光計 | 1セット | – | リード中空陰極ランプ |
| 2 | 空気圧縮機 | 1セット | 評価された放電圧力: 0.3 MPa | – |
| 3 | アセチレンガス | 1つのシリンダー | 純度 ≥ 99.99% | – |
2.1.2 サンプル前処理装置
| シリアル番号 | 名前 | 量 | 技術要件 | アクセサリー |
|---|---|---|---|---|
| 1 | 電子バランス | 1セット | 感度: 0.1 mg | – |
| 2 | ビーカー | いくつかの | 容量: 150 mL | – |
| 3 | 調節可能なホットプレート | 1セット | 温度範囲: 室温〜300℃ | – |
| 4 | 分離ファネル | いくつかの | 容量: 250 mL | – |
| 5 | マイクロピペット | 各1枚 | 範囲:100μL〜1000μL、1000μL〜5000μL | – |
| 6 | 色計チューブ | いくつかの | 容量: 10 mL | – |
2.2 試薬
2.2.1 原試薬
| シリアル番号 | 名前 | 技術要件 | 備考 |
|---|---|---|---|
| 1 | 4-メチル-2-ペンタノン(MIBK) | 分析試薬(AR) | – |
| 2 | アンモニア 水 | 分析試薬(AR) | – |
| 3 | 硫酸アンモニウム | 保証された試薬(GR) | – |
| 4 | シトレートアンモニウム | 分析試薬(AR) | – |
| 5 | ディエチルディチオカーバメートナトリウム(DDTC) | 保証された試薬(GR) | – |
| 6 | ブロモチモール ブルー | 分析試薬(AR) | – |
| 7 | 硝酸 | MOS等級 | – |
2.2.2 準備された試薬
| シリアル番号 | 名前 | 準備方法 | 備考 |
|---|---|---|---|
| 1 | アンモニア水溶液 (1 1) | 50 mLのアンモニア水を測定し、50 mLの水に加え、よく混合します。 | – |
| 2 | 硫酸アンモニウム溶液(300 g/L) | 硫酸アンモニウム30gを重量し,水に溶解し,水で100mLまで硫酸硫酸アンモニウムを硫酸溶解します. | – |
| 3 | シトレートアンモニウム溶液(250 g/L) | シトレートアンモニウム25gを重量し,水に溶解し,水で100mLまで水水溶解します. | – |
| 4 | ディエチルディチオカーバメートナトリウム(DDTC)溶液(50 g/L) | 5 gのディエチルディチオカルバメートナトリウムを重量し,水に溶解し,水で100 mLまで水水溶解します. | – |
| 5 | ブロモチモールブルーインディケーターソリューション (1 g/L) | ブロモチモールブルーの0.1gを重み,水に溶け,水で100mLまで水水水でブブブロモチモールブルーをブブブロモチモールブルーを0.1gを量し,水で溶解し,水で100mLまでブブブ | – |
| 6 | 硝酸溶液(5+95) | 硝酸50 mLを計量し、水950 mLに加えてよく混ぜます。 | – |
2.3 参照基準
2.3.1 ストックソリューション
| シリアル番号 | いや | 名前 | 技術要件 | 備考 |
|---|---|---|---|---|
| 1 | GSB G 62071-90 | リード標準ソリューション | 浓度: 1000 μg/mL | 中央鉄鋼研究所,国立鉄鋼材料試験センター |
3. 結果計算
リード含有量は質量分率で表されます W 铅(Pb)の単位(mg/kg)は、次の式で計算されます。
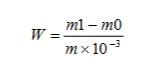
どこ:
- m1=作業曲線から得られた試験溶液の作作業曲線の作作単位:ミリグラム(mg)
- m0 =作業曲線から得られた空白試験溶液の作作作作作業曲線から得られた作作業曲線から得られた作作作業曲線から得られた作作作業曲線から得られた空白試験溶液の作業単位の
- m サンプルの質量、単位:グラム(g)。
平行決定結果の算術平均値は最終決定結果として取られる。2つの並行決定結果の絶対差は1mg/kgを超えないでください。

