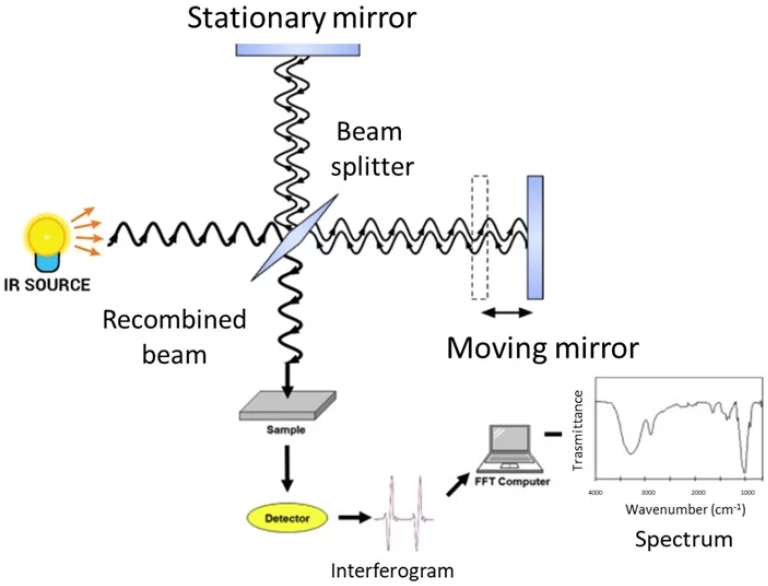
الكروماتوغرافيا الغازية هي تقنية لفصل المكونات عن المادة الخام عن طريق مرحلة متنقلة غازية. أصبحت هذه التقنية التحليلية شائعة جدا في فصل وتحديد وقياس المركبات المتطايرة وشبه المتطايرة في الخليطات المعقدة. تتضمن العملية الأساسية تسخين العينة إلى بخار ، ونقلها عبر عمود عن طريق غاز خامل ، مما يساعد على الفصل بناءً على خصائص فيزيائية كيميائية محددة. أصبحت GC جوهرية في العديد من المجالات التي تتراوح من المراقبة البيئية إلى ضمان جودة الأدوية بسبب الفعالية والحساسية والموثوقية التي توفرها.
مبادئ فصل الكروماتوغرافيا الغازية
تشير الكروماتوغرافيا الغازية إلى مختلف طرق الفصل التحليلي المطبقة لمراقبة المواد المتطايرة في الحالة الغازية. في الكروماتوغرافيا الغازية، يتم ذوبان مكونات عينة أولا في بعض المذيبات. ثم يتم تبخيرها. هذا هو فصل التحليلات التي يتم إنجازها عن طريق نشر العينة بين مرحلتين: مرحلة ثابتة ومرحلة متنقلة. المرحلة المتنقلة ، عادة غاز خامل مثل الهيليوم أو النيتروجين ، تدفع العينة المبخرة عبر عمود. يتم تغطية العمود داخليا ببعض المرحلة الثابتة السائلة أو الصلبة. بما أن المركبات تتفاعل مع هذه المرحلة الثابتة بشكل مختلف ، فإنها تخرج في أوقات الاحتفاظ المختلفة.
المكونات الرئيسية لنظام الكروماتوغرافيا الغازية
يحتوي الكروماتوغراف الغازي على عدة أجزاء رئيسية:
- غاز الناقل: عادة ما يكون هذا الهيليوم أو النيتروجين أو الهيدروجين ، ويعمل كمرحلة متنقلة.
- حقن: هذا الجزء يبخر العينة. ثم يتم إدخالها في العمود.
- عمود: يمكن أن تكون إما معبأة أو شعرية. ما’ أكثر من ذلك ، فإنه يفصل المركبات بناء على خصائصها الكيميائية.
- كاشف: يستخدم لقياس تركيز المركبات عندما تترك العمود.
يتم الاحتفاظ بالأجزاء التحليلية من الكروماتوغراف الغازي داخل الفرن حيث يتم التحكم بدقة في درجة الحرارة.
الآليات وراء فصل المواد الكيميائية في GC
تتم توجيه فعالية الفصل الكيميائي في الكروماتوغرافيا الغازية من خلال الخصائص الطبيعية المختلفة للمحللات وظروف الأداة.
دور التقلب في كفاءة الفصل
المركبات ذات نقاط غليان أقل أكثر تقلباً. لذلك ، فهي تخرج بشكل أسرع. بينما تسافر عينة الغاز عبر العمود ، تمر المكونات ذات نقاط غليان أقل بسرعة أكبر من تلك ذات نقاط غليان أعلى. تتيح هذه الفكرة الفصل القائم على الوقت حيث يظهر كل مركب كذروته الخاصة على الكروماتوغرام.
تأثير الاستقطاب وتفاعلات المرحلة الثابتة
تفاعل قطبية المركب مع كيمياء المرحلة الثابتة يؤثر بشكل كبير على وقت الاحتفاظ. تتفاعل المركبات القطبية بقوة أكبر بكثير مع المراحل الثابتة القطبية ، مما يزيد من وقت الاحتفاظ. وبالتالي ، سيؤدي اختيار مرحلة ثابتة ذات قطبية مشابهة للمحلل إلى تحسين الدقة والانتقائية.
برمجة درجة الحرارة وتأثيرها على الفصل
التحكم في درجة الحرارة ضروري للفصل القابل للتكرار. منذ كروماتوغراف الغاز’ يتم استضافة الأعمدة والكاشف في نفس الفرن ، ويرتبط أداء الأداة مباشرة باستقرار درجة الحرارة. وبالتالي ، عادة ما تبقى درجة الحرارة ثابتة إلى ± 0.5 درجة فهرنهايت (± 0.3 درجة مئوية). عندما تحتوي العينة على مكونات ذات مجموعة واسعة من نقاط الغليان ، فإن برمجة درجة الحرارة - التي تزيد فيها درجة حرارة الفرن بمعدل مسيطر عليه - توفر فصل أفضل عن القمم وتتطلب وقت تحليل أقل.
أنواع الأعمدة المستخدمة في الكروماتوغرافيا الغازية
تصميم العمود يؤثر فعلا على أداء GC. لذلك ، يجب اختيارها بناء على أهداف التحليل.
الأعمدة المعبأة مقابل الأعمدة الشعرية
الأعمدة المعبأة مليئة بدعم صلب خامل مغطى في مرحلة ثابتة. إنها جيدة لأحجام عينة أكبر لكنها توفر دقة أقل حدة. على النقيض من ذلك:
الأعمدة الشعرية (التي تسمى أيضًا الأعمدة الأنابيبية المفتوحة) لها قطر داخلي صغير جداً. يتم تغطية داخليها بمرحلة ثابتة. هذا يوفر كفاءة أعلى ويخلق قمم أكثر حدة بكثير. في عمود شعري ، يتم تطبيق طبقة رقيقة من المرحلة الثابتة مباشرة على الجدران الداخلية للأنابيب.
اختيار العمود المناسب لتطبيقك
يعتمد القرار بين هذه الأنواع على أشياء مثل تعقيد العينة ، ومقدار الدقة التي تحتاجها ، ومدى سرعة التحليل ، والمعدات التي لديك. لتحليل الآثار التي تحتاج إلى دقة عالية جدا، الأعمدة الشعرية هي دائما الخيار الأفضل تقريبا.
العوامل التي تؤثر على الفصل الكيميائي في تحليل GC
للحصول على أفضل فصل كيميائي، يجب التحكم في بعض إعدادات التشغيل بعناية كبيرة.
اعتبارات معدل تدفق الغاز الناقل
تؤثر سرعة الغاز الناقل على وقت التحليل والدقة. هذا’ هو عمل توازن. معدل تدفق أعلى يجعل الأمور أسرع ولكن قد يسبب تداخل القمم. من ناحية أخرى ، يمكن لمعدل التدفق البطيء جدا أن يجعل القمم أوسع. وبالتالي، فإن معدل التدفق المثالي يجد توازنا جيدا بين السرعة وجودة الفصل.
تقنيات حقن العينات وآثارها على النتائج
أنظمة GC الحديثة لها كل من أوضاع الحقن المقسمة وغير المقسمة:
- حقن تقسيم يحافظ على العمود من الحصول على التحميل الزائد. وهو يفعل ذلك عن طريق السماح فقط في جزء من العينة.
- حقن غير مقسم يقدم العينة بأكملها. هذا للكشف عن المواد في مستويات منخفضة جدا.
- غالبا ما تسمح لك أجهزة الكروماتوغراف الغازية التجارية باستخدام كل من الحقن المنقسمة وغير المنقسمة ، وهو أمر مفيد عند التبديل بين الأعمدة المعبأة والأعمدة الشعرية.
طول العمود والقطر ومعلمات سمك الفيلم
أعمدة أطول تعطيك فصل أفضل. ومع ذلك ، فإنها تجعل وقت التشغيل أطول. الأقطار الداخلية الأضيق تحسن الكفاءة ولكن تحتاج إلى ضغط أكبر للعمل. وأخيرا، تزيد طلاءات الأفلام السميكة من وقت الاحتفاظ بالمحللات المتطايرة، مما يساعد على تحسين دقة الذروة.
طرق الكشف بعد الفصل الكيميائي
بمجرد فصل المركبات بوقت الاحتفاظ بها ، يجب الكشف عنها بدقة. كما يجب قياسها كمياً بالكاشفات المناسبة.
كاشفات شائعة تستخدم في أنظمة GC
عدة كاشفات شائعة جدا في أدوات GC:
- كاشف أيونة اللهب (FID)
عادة ما يكون FID هو الخيار الأكثر ملاءمة بسبب حساسيته والقرار الكبير. كما أنه جيد لأنه يمكن أن يكتشف جزيئات صغيرة جدا. وهو مفيد بشكل خاص لتحليل الهيدروكربونات بسبب نطاقه الديناميكي الواسع. - كاشف التوصيل الحراري (TCD)
يلاحظ TCD تغييرات في الموصلية الحرارية بين الغاز الناقل وغازات التحليل. يمكن أن يكتشف أي شيء ولكن ليس حساسًا مثل FID. - كاشف التقاط الإلكترونات (ECD)
ECD حساس للغاية للمركبات التي تحتوي على الهالوجينات. وهو مثالي لمراقبة البيئة التي تنطوي على أشياء مثل مبيدات الآفات أو المبردات.
تطبيقات الكروماتوغرافيا الغازية عبر الصناعات
لأنها مرنة للغاية ، أصبحت الكروماتوغرافيا الغازية ضرورية في العديد من المجالات المختلفة.
رصد البيئة ومكافحة التلوث
يمكنك تحليل عينات الهواء باستخدام GC. يستخدم كثيرا للعثور على المركبات العضوية المتطايرة (VOCs) في الهواء والماء والتربة. بالإضافة إلى ذلك ، تستخدم الوكالات التنظيمية GC للتحقق من الانبعاثات الصناعية والتأكد من اتباعها للقواعد البيئية.
مراقبة الجودة الصيدلانية
الكروماتوغرافيا الغازية تساعد على الحفاظ على الأدوية آمنة. يقوم بذلك عن طريق اختبار نقاء المكونات الصيدلانية النشطة (API). كما يحدد أي مذيبات متبقية بعد التوليف.
اختبار سلامة الأغذية
يمكن لـ GC اكتشاف بقايا المبيدات الحشرية في المحاصيل. كما يمكن أن يكتشف عوامل النكهات أو الملوثات في الأغذية. تساعد هذه الفحوصات على التأكد من أن الأطعمة تلبي المعايير الصحية.
PERSEE: مصنع موثوق للأدوات التحليلية
برسي يقدم حلول متقدمة للتحليل الكيميائي. لهم نموذج G5GC يوفر الفصل عالي الأداء الذي يتم إنشاؤه للمهام المعقدة ، في حين أن سلسلة M7 يضع الكروماتوغرافيا الغازية في منصات متعددة الوظائف. هذه هي رائعة للمختبرات التي تحتاج إلى المرونة ولكن يمكن’ t تحمل فقدان الدقة.

الالتزام بالابتكار والجودة والدعم العالمي
تركز شركة PERSEE على الهندسة القوية. كما أنها تعطي الأولوية للواجهات السهلة الاستخدام والمساعدة التقنية في جميع أنحاء العالم. شبكة التوزيع العالمية الخاصة بهم تجعل من السهل الحصول على أنظمة GC المتقدمة في الإعدادات الأكاديمية والصناعية والبحثية في جميع أنحاء العالم.
ملخص المفاهيم الرئيسية في الفصل الكروماتوغرافي للغاز
الفصل الكيميائي في الكروماتوغرافيا الغازية ينخفض حقا إلى بعض الأشياء. هذه هي الاختلافات في التقلب، تفاعلات الاستقطاب، اختيار العمود، والحصول على معدل التدفق الصحيح، والتحكم في درجة الحرارة. يضمن اختيار الكاشف الصحيح لك قياس الكميات بدقة عبر جميع أنواع أنواع العينات المختلفة.
أهمية تحسين الطريقة
يتطلب تطوير طريقة تعديل دقيق لتقنية الحقن واختيار غاز الناقل وبرمجة درجة الحرارة وتوافق الكاشف. يتم كل هذا للحصول على نتائج موثوقة وقابلة للتكرار في كل تطبيق واحد.
الأسئلة الشائعة:
Q1: هل يمكن استخدام الكروماتوغرافيا الغازية لفصل المركبات غير المتطايرة؟
A1: لا. الكروماتوغرافيا الغازية هي فقط للمركبات المتطايرة أو شبه المتطايرة التي يمكن أن تتحول إلى بخار دون تحلل. تحتاج المواد غير المتطايرة إلى طرق مختلفة ، مثل الكروماتوغرافيا السائلة.
س2: كيف أختار بين كاشفات FID و TCD؟
A2: FID أفضل للكشف عن الهيدروكربونات لأنه’ حساسية جداً. TCD هو أكثر من كاشف الغرض العام ولكن ليس حساسًا. لذلك, it’ خيار جيد عندما تكتشف الغازات غير العضوية أو عندما لا يكون الكاشف القائم على اللهب خيارًا.
س3: لماذا تتداخل بعض القمم حتى بعد ضبط درجة الحرارة؟
A3: يمكن أن تحدث قمم متداخلة إذا كان اختيار العمود سيئًا أو إذا كان القرار ’ جيد بما فيه الكفاية؛ محاولة مرحلة ثابتة مختلفة أو ضبط معدل التدفق يمكن أن تساعد في تحسين أداء الفصل.
Q4: هل الكروماتوغرافيا الغازية مناسبة لمراقبة جودة الهواء في الوقت الحقيقي؟
A4: نعم. الكثير من وحدات مراقبة جودة الهواء تستخدم GC جنبا إلى جنب مع كاشفات FID أو ECD. يقومون بذلك لمراقبة مستويات المركبات العضوية الجوية في الوقت الحقيقي بسبب حساسيتها العالية ودقتها.
س5: ما هي أنواع العينات التي يمكن لأدوات GC PERSEE تحليلها؟
A5: أنظمة GC من PERSEE مصنوعة لمجموعة واسعة من الاستخدامات. وتشمل هذه الأدوية والعينات البيئية والبتروكيماويات واختبارات سلامة الأغذية والبحوث الأكاديمية ، كل ذلك بفضل إعداداتها القابلة للتخصيص.